Clinical Trials: What does the future hold?
The clinical trial landscape is undergoing a fundamental transformation, as we emerge from the pandemic. Traditional processes are embracing new formats to reinvent clinical developments to harness a more patient-centric approach, through advancements in research and development, digital modernisation, and technology.
Clinical trials are one of pharmaceuticals most costly processes, costing on average around $19 million each. So, how are game changing innovations are shaping better, faster, and cheaper drug development? Let’s deep dive into the rising tide of change in clinical trials:
Adopting the use of XR
Immersive technologies are breaking the mould when it comes to therapeutic capabilities. Virtual reality technology is being used to change the way patients process chronic and acute pain, as well as anxiety by developing new, positive habits and coping skills.
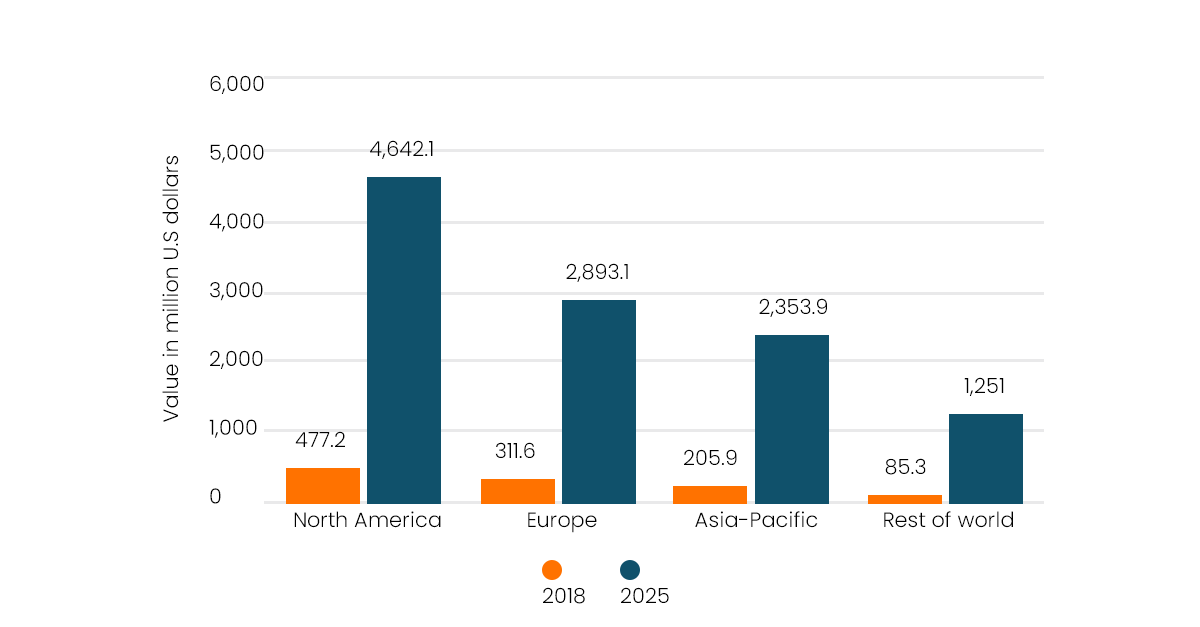
XR (Extended Reality) is a pioneering patient-centric tool that can be utilised to not only treat patients’ pain and mental health, but also to help patients understand their treatment plan and procedures as well as explain their condition in detail. In 2018, the European healthcare AR and VR industry was valued at $311 million. By 2025, this is expected to increase drastically to $2.89 billion. Yes, you heard that right! As costs of technologies to us end users plummet and innovative ways to use XR are discovered, the use of AR and VR in the medical sector will only continue to rise.
Harness the power of data
Wearable technology is booming, with 30% of UK adults currently owning and using a wearable device. It’s more than likely you are wearing one now, as an abundant amount of us are obsessed with tracking our exercise, heart rate and sleep patterns. The medical industry is now leveraging built-in sensors in patients’ clothing, devices, or even specific parts of their body (crazy), to capture and collect effortless data, in a more efficient and convenient way than ever before.
Researchers have designed a temporary tattoo that’s pioneering the field of atomically thin bioelectronics that are wearable. Unlike your ink tattoo, this graphene-based tattoo functions as wearable technology. This innovative ink is being used to monitor ECG measurements, drug release and fall detection.
Wearable technology is providing continuous, real-time data to curate a holistic overview of a patient’s health in clinical trials. The data will help to provide a more accurate and deep understanding of the direct impact that clinical trials have on a patient’s health and environment when they are receiving their treatment.
Intelligent digital agents
Clinical trials are time consuming, expensive, and burdensome on patients. Studies show that 80% of trials are delayed by at least a month due to not meeting enrolment timelines. Before a clinical trial can be conducted, specific patients must be enrolled and go through a timely process before they are successful.
Doctors in the future will gain access to intelligent digital platforms that will select appropriate trials based on patient data. AI and machine learning will power the digital platforms, to automatically check if a patient is eligible before the onboarding process begins.
To leverage these digital agents, all parties of clinical trials must use paperless systems. As AI advances, the technology will be able to conduct complex tasks at mega speed. This ground-breaking technology will facilitate fast and efficient trial discovery and a simpler process of onboarding patients.
Stay at home trials
Frequent visits to clinics will soon become a thing of the past. Pioneering advancements will be the catalyst of providing patient-centric trials, in a time and location more convenient, such as the comfort of their own home, no matter their geographic location. Science 37 is taking on this challenge by reinventing the clinical trial, from hybrid to fully decentralised.
Science 37 is creating universal access to clinical research, allowing patients and providers to participate from anywhere, which is resulting in the accelerated development of treatments. The Science 37 Clinical Trial Operating System uses a decentralised and Metasite clinical trial research design through their end-to-end technology platform, which includes:
- Specialised networks of patient communities
- Telemedicine investigators
- Community providers
- Mobile nursing
- Remote coordinators
- Connected data and devices
Science 37 states that their technology enables up to a 15x faster enrolment, 28% better retention and 3x more diverse patient population. Leveraging unified technology, standardised processes, and centralised networks will accelerate breakthrough clinical research.
Could companies such as these be setting the precedent for the future of clinical trials?
Personalised trials
Precision medicine is an emerging approach in clinical trials, which takes into consideration each individual patients’ genes, environment, and lifestyle. The research and advances of predictive modelling within healthcare data are expected to drive the use of personalised medicine. Having access to patient specific information, will help clinicians curate in-depth accurate knowledge of a patient’s environment and history whilst modifying individual treatments based on deeply informed predictive models.
Personalised trials that are tailored to each individual patient should minimise or even eliminate any negative side effects, leading to improved outcomes for each patient. Personalised trials will pioneer treatment or condition research by providing deep insights into patient specific variability.
The future of clinical trials
So, what does this vision of the future mean for the pharmaceutical industry? It’s evident that adopting and applying ground-breaking technology is essential for success of the next evolution of clinical trials. Innovative technology will be a catalyst of positive change for all parties that are involved in each clinical trial process, making them more efficient and cost-effective.
Our latest whitepaper Entering the GreenTech Era was a cracking read, but we are already jumping straight on to the next. Our upcoming whitepaper will cover everything you need to know about new developments in the MedTech landscape, so keep an eye out to stay in the know.
